Dealing with tapeworm infections in horses in the UK (2024)
Prof. Jacqui Matthews, BVMS PhD FRSE FRSB FRCVS, RCVS Specialist in Veterinary Parasitology, Director of Veterinary Science, Austin Davis Biologics
Horses are infected by a range of parasitic worms whilst grazing. Unless excellent pasture hygiene is practiced, infection is unavoidable. Anoplocephala perfoliata is the commonest equine tapeworm. Infection occurs following ingestion of forage contaminated with oribatid mites that contain tapeworm larvae. Larvae are released in the intestine where they develop to adults near the small-large intestinal junction. Adult tapeworms are hermaphrodite and generate egg-containing segments, which are released at irregular intervals. The segments disintegrate as they move down the intestine. Once in the environment, eggs can be eaten by oribatid mites that encounter eggs by chance. Within mites, larvae hatch and develop to infective stages in 8-20 weeks. Infected mites can persist on paddocks for extended periods.
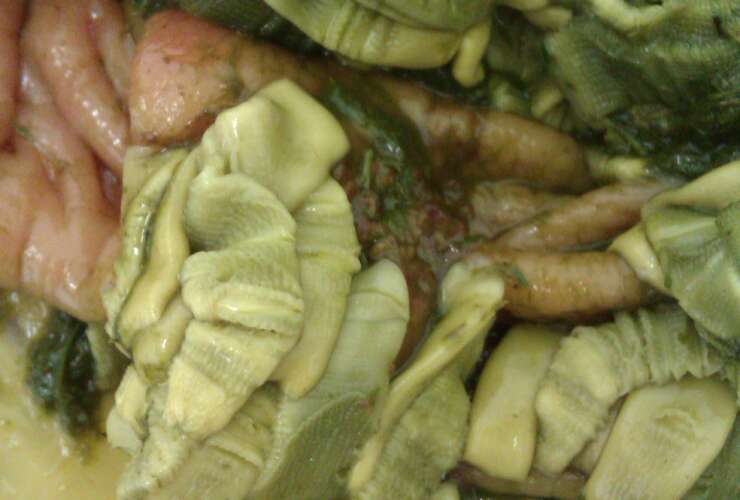
Horses of all ages can harbour tapeworm [1], with infections occurring year-round [2]. Across populations and studies, the proportion of horses infected with tapeworm varies from <10% to >80% [3], meaning that, without diagnostic testing, it is difficult to establish whether or not a horse is infected. Often, individuals harbour <100 tapeworms [4], but it does not take many tapeworms to cause injury. Burdens of >20 worms have been shown to cause damage [5] which can result in colic and is associated with infection intensity [6-8]. It is therefore important to identify which horses have higher levels of tapeworm so that they can be treated with an appropriate anthelmintic to reduce the risk of disease.
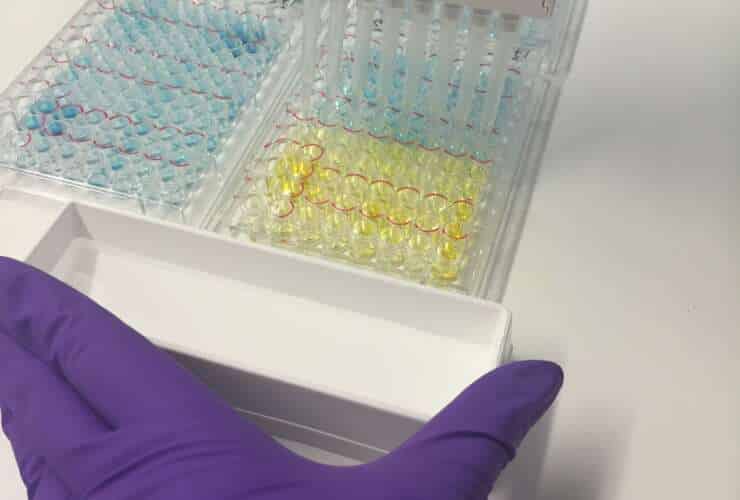
Tapeworm-infected horses can be identified by testing. Because tapeworms shed egg-containing segments intermittently [9], with infections often comprising proportions of immature/sterile worms [10], coprology methods are insensitive for detecting infection. Antibody testing is a suitable method for detecting tapeworm infection, with options available in blood or saliva formats. In these tests, the level of tapeworm-specific antibody measured is associated with the level of burden present [11;12]. Test results are therefore categorised as ‘low’, ‘borderline’ and ‘moderate/high’, with anti-tapeworm treatment advised for horses that report in the last two categories. When used to provide information on infection, antibody testing can result in substantial reductions in anthelmintics use; in the UK, 2 out of every 3 horses assessed using the saliva test (EquiSalTM, Austin Davis Biologics) do not require treatment. It is important to reduce over-use of wormers as resistance to anti-tapeworm compounds (pyrantel, praziquantel) was recently reported [13]. With resistance already prevalent in nematodes such as small redworms and ascarids, this means that this is now an issue in all common equine worms.
Tapeworm control needs to be part of an overall programme designed to target all types of worms. Control plans must include excellent pasture management (e.g., fully removing dung at least twice-weekly, maintaining low stocking densities) to reduce environmental infection. Lower paddock contamination will lead to reduced worm burdens, resulting in less animals being positive in tests and so less treatments are applied. This will reduce selection pressure for wormer resistance. Testing enables selection of the correct type of wormer. For example, if there is no indication for anti-nematode treatment, a product specific for tapeworm should be used to avoid unnecessary selection for resistance. In such cases, combination products should be avoided and a narrow-spectrum product chosen. Praziquantel is an anti-tapeworm anthelmintic that will not exert a selection effect on nematode species. In the UK, praziquantel is available as an extemporaneous formulation which can be prescribed by veterinary surgeons in accordance with the cascade (The cascade: prescribing unauthorised medicines – GOV.UK (www.gov.uk))

References
- Sallé et al. 2020. Int J Parasitol. 50, 125-132.
- Engell-Sørensen et al. 2018. Vet Parasitol: Reg Stud Rep. 12;22-25.
- Matthews et al. 2023. Pathogens 12:1233.
- Tomczuk et al. 2014. Parasitol Res. 113, 2401-2406.
- Pavone et al. 2010. Vet. Res. Commun. 34 Suppl 1, S53-6.
- Proudman & Edwards, 1993. Equine Vet J. 25, 224-226.
- Proudman et al. 1998. Equine Vet. J. 30, 194-199.
- Veronesi et al. 2009. Vet Res Commun. 33 S1, 161-163.
- Gasser et al. 2005. Parasitology 131, 1-13.
- Meana et al. 2005. Vet. Parasitol. 130, 233-240.
- Proudman & Trees, 1996. Parasite Immunol. 18, 499-506.
- Lightbody et al. 2016. Vet Clin Pathol. 45;335-346.
- Nielsen, 2023. Int J Parasitol Drugs Drug Resist. 22, 96-101.




